FDA Authorizes 642 Revisions to CoV-2 Emergency Use Authorizations: Adding 415 EUA CoV-2 Testing Devices
In an FDA October coronavirus press release, 642 revisions were made to Emergency Use Authorizations. Additionally, 415 tests and sample collection devices were authorized by the FDA under emergency use authorizations (EUAs).
While it may seem like good news that 415 new tests exist, this move, [along with the 642 revisions of Emergency Use Authorizations] indicates that the FDA may have financially incentivized priorities other than fully completing testing before releasing such devices.
These testing devices will be used to provide data to the American public, and regulate the state. Should coronavirus testing devices be fully tested and authorized before treating the data collected for these devices as fact?
At first glance these new testing devices appear to be in the interest of the American public, providing more access to testing for the virus. The truth is that these tests literally define what is considered “misinformation”. These tests are contributory of the modern divide of the American population, and the Big Tech suppression of competing data and alternate information. Shouldn’t coronavirus tests be fully tested before relying on and controlling the order of our modern society?
415 Tests and Sample Devices Authorized under EUA
The 415 coronavirus tests and sample collection devices that were authorized by the FDA for use under emergency use authorizations (EUAs) include,
289 Molecular tests and sample collection devices
89 Antibody and other immune response tests
36 Antigen tests
65 Molecular authorizations and
1 Antibody authorization [to be used with home-collected samples.]
1 Molecular prescription at-home tested
3 Antigen prescription at-home tests
8 Antigen over-the-counter (OTC) at-home tests
2 Molecular OTC at-home tests.
[The FDA also authorized 16 antigen tests and eight molecular tests for serial screening programs.]
Read the entire press release here
Emergency Use Authorization
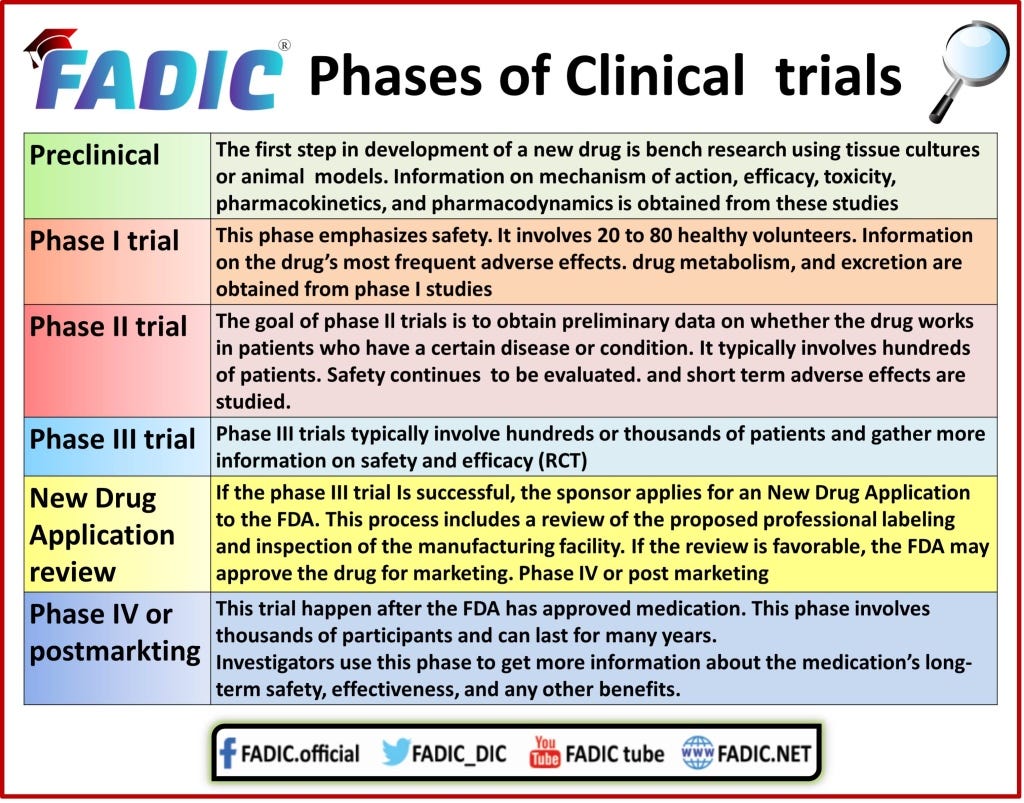
Emergency Use Authorizations (EUA) can be given at anytime after Phase 3 Clinical Trials.
“Under section 564 of the Federal Food, Drug, and Cosmetic Act (FD&C Act), when the Secretary of HHS declares that an emergency use authorization [EUA] is appropriate, [the] FDA may authorize unapproved medical products or unapproved uses of approved medical products to be used in an emergency to diagnose, treat, or prevent serious or life-threatening diseases or conditions caused by CBRN threat agents when certain criteria are met, including there are no adequate, approved, and available alternatives.”
“The HHS declaration to support such use must be based on one of four types of determinations of threats or potential threats by the Secretary of HHS, Homeland Security, or Defense.”
Previously Faulty PCR Tests
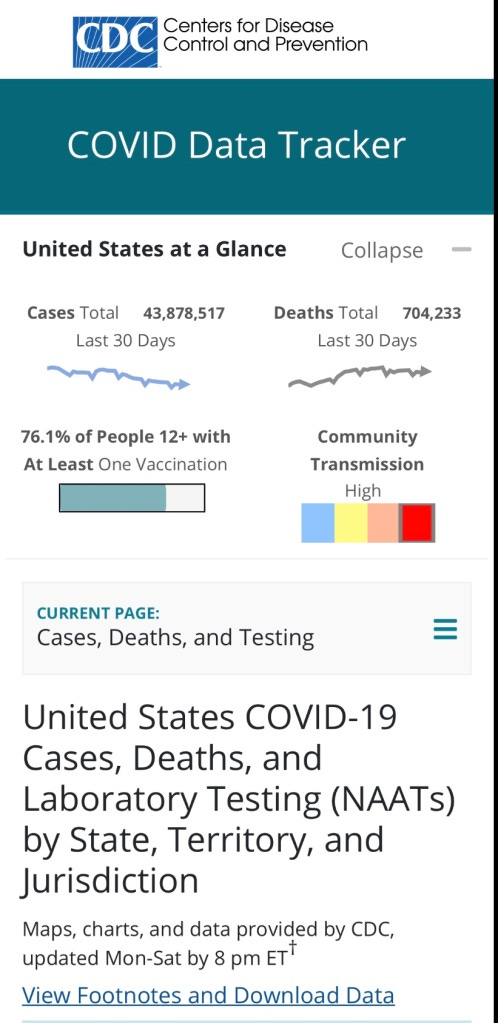
The FDA works with the CDC to regulate the tests that are performed across America to gather information to populate the real-time information depicted on their official website. This data is used to create guidelines that control whether or not businesses are allowed to operate, and citizens are allowed to congregate.
Data indications shown on the CDC’s official website use tests “authorized” by the FDA. How accurate is this data, and how much have these “tests” contributed to “misinformation”?
The FDA previously issued faulty PCR tests under Emergency Use Authorization to the American public, that influenced COVID-19 statistics, and state-to-state lockdowns during of the initial outbreak of coronavirus pandemic. This recall was for the SARS-CoV-2 Antigen Rapid Qualitative Test manufactured between September 2020 – March 2021. The tests were issued a Class I recall, defined by the FDA as, “the most serious type of recall. Use of these devices may cause serious injuries or death.”
Conclusion
Now, with 642 “revisions” created, and 415 “new” coronavirus tests “authorized”, how can the American population trust the regulations set in place by the data accumulated from these testing devices?
Update***Originally displaying “641” and “414”, on October 8th, 2021 the FDA added 1 more revision, and 1 more device. The headline and contents of this article was adjusted to display this update.